Antibody-Drug Conjugates (ADCs) have been discussed for their therapeutic potential for over a century.
However, in the last 10 years, the field has gained momentum with the clinical approval of 13 drugs (see table below) with more expected to follow. All these drugs share one common feature, the targeting vehicle is a whole monoclonal antibody (mAb, IgG) with a molecular weight of 150 kDa, the variables are the conjugation chemistry used, the drug-loading and the combination of linker-drug (payload).
Trade Name | Approval Year | Drug | Payload |
Mylotarg | 2017 | Gemtuzumab ozogamicin | Gemtuzumab ozogamicin |
Adcetris | 2011 | Brentuximab vedotin | Brentuximab vedotin |
Kadcyla | 2013 | Trastuzumab emtansine | Trastuzumab emtansine |
Besponsa | 2017 | Inotuzumab ozogamicin | Inotuzumab ozogamicin |
Lumoxiti | 2018 | Moxetumomab pasudotox | Moxetumomab pasudotox |
Polivy | 2019 | Polatuzumab vedotin-piiq | Polatuzumab vedotin-piiq |
Padcev | 2019 | Enfortumab vedotin | Enfortumab vedotin |
Enhertu | 2019 | Trastuzumab deruxtecan | Trastuzumab deruxtecan |
Trodelvy | 2020 | Sacituzumab govitecan | Sacituzumab govitecan |
Blenrep | 2020, withdrawn November 2022 | Belantamab mafodotin-blmf | Belantamab mafodotin-blmf |
Zynlonta | 2021 | Loncastuximab tesirine-lpyl | Loncastuximab tesirine-lpyl |
Tivdak | 2021 | Tisotumab vedotin-tftv | Tisotumab vedotin-tftv |
ELAHERE | 2021 | Mirvetuximab soravtansine | Mirvetuximab soravtansine |



SYNthesis med chem Capabilities
For the past five years, SYNthesis med chem scientists have been involved in several projects related to Antibody Drug Conjugates, working alongside many different clients with different specifics to develop novel ADCs that incorporate innovative mechanisms of action. Our main focus has been on synthesizing different linkers and payloads /warheads.
Focusing on the synthesis of linker payloads/warheads
We have provided our clients with various payloads, linkers and conjugatable “building blocks”, allowing them to design and assess the potency, efficacy, and pharmacological properties of potential ADCs. Our scientists are highly skilled in the design and synthesis of a variety of payload and linkers, and are fully supported by our state of the art analytical facility.
PAYLOADS
We have successfully prepared a range of payloads, covering several mechanisms of action including microtubule polymerization inhibitors and DNA binders. Our bench chemists are fully trained to handle these cytotoxic warheads, which allow us to not restrict our offer to non cytotoxic warheads
Example of payloads we worked on:
- Auristatins (e.g. MMAE, MMAF)
- Pyrrolobenzodiazepines, Indolinobenzodiazepine and their derivatives
- Duocarmycins and its derivatives
- Camptothecin (e.g. SN38)
- Some other innovative drugs designed by our collaborators



LINKERS
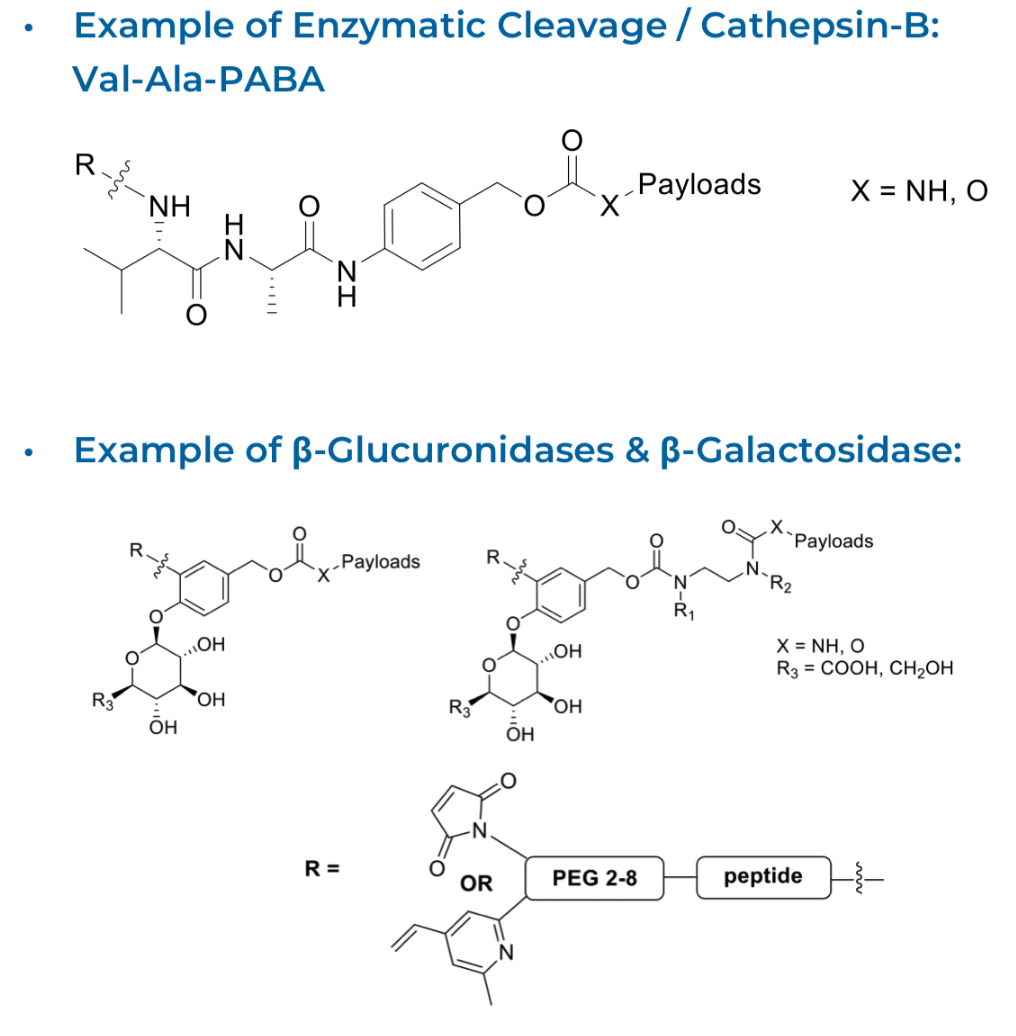
We have synthesised a range of cleavable linkers, based around peptides, glucuronides, disulfides and thioethers which are processed intracellularly to release the warhead or payload.
We have prepared a range of non-cleavable linkers based around peptide, carbohydrate, thioether and PEG components. These cleavable and non cleavable linkers have been readily attached to payloads or warheads, and have further incorporated reactive conjugatable groups that allow for lysine, cysteine and non-natural amino acid conjugation.


Our other services
Can’t see what you’re looking for? Please get in touch
We love to talk about Discovery Chemistry
Email or fill in the form opposite and our dedicated team will get back to you as soon as possible.
E. enquiries@synmedchem.com